How Many Paired Electrons Are in Helium
Each of the individual f-orbitals can hold 2 electrons each. How many electrons are transferred in the balanced reaction ie what will be the value of n in the Nernst equation.

Aufbau Principle Electrons Enter The Lowest Energy First Ppt Download
010 M MnO4- 040 M Cr3 020 M Mn2 030 M Cr2O72-0010 M H 0010 M H The standard reduction potentials are as follows.

. A nuclide is a species of an atom with a specific number of protons and neutrons in the nucleus for example carbon-13 with 6 protons and 7 neutrons. The nuclide concept referring to individual nuclear species emphasizes nuclear properties over chemical properties whereas the isotope concept grouping all atoms of each element emphasizes. Refer to the galvanic cell below the contents of each half-cell are written beneath each compartment.
An ion ˈ aɪ ɒ n-ən is an atom or molecule with a net electrical charge. When l 3 the orbitals are called f-orbitals and there are 7 of them. This gives a total of 10 electrons that can go into the 5 d-orbitals.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton which is considered to be positive by conventionThe net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. This gives a total of 14 electrons that can go into the 7 f-orbitals. Each of the individual d-orbitals can hold 2 electrons each.
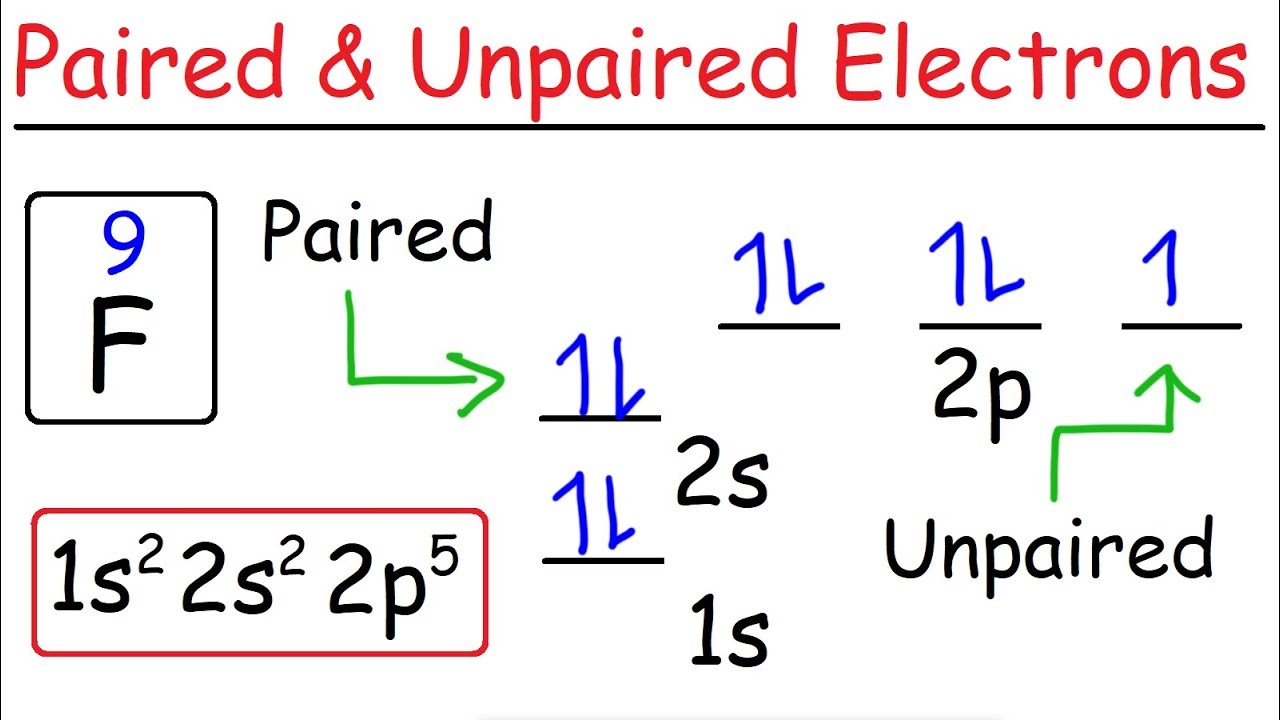
How To Determine The Number Of Paired And Unpaired Electrons Youtube

Helium He Electron Configuration And Orbital Diagram

No comments for "How Many Paired Electrons Are in Helium"
Post a Comment